Uterine fibroids (also known as leiomyomas or myomas) consisting of smooth muscle and a variable amount of connective tissue, are the most common benign solid tumors of the female genital tract, affecting 20–25% of women of reproductive age.
The myoma initially grows inside the myometrium and then tends, displacing the surrounding myometrial fibers, to move towards areas of less resistance: the abdominal cavity or the uterine cavity.
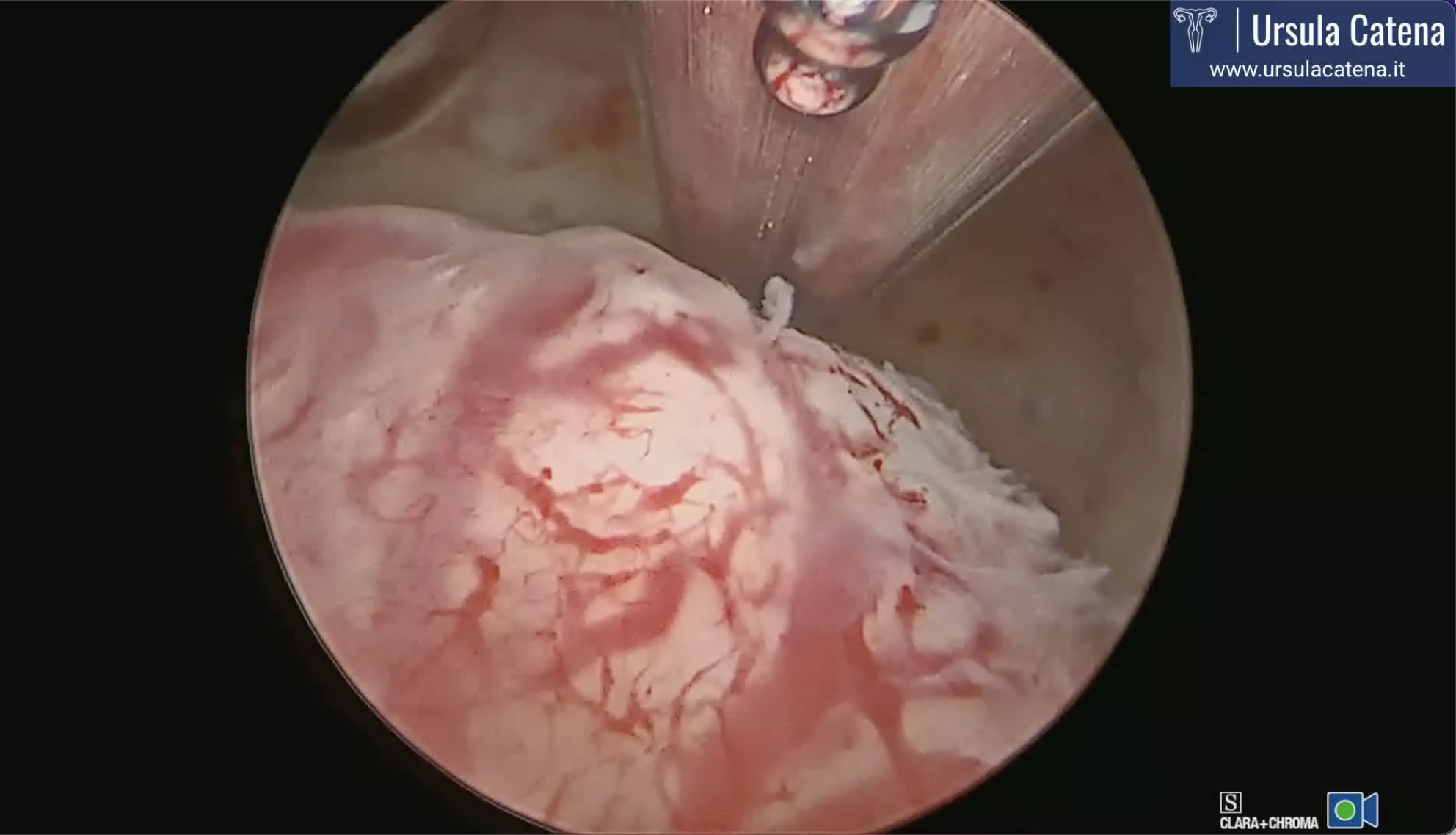
Myomas can therefore be classified, based on their location, in: submucosal (10%), when they protrude into the uterine cavity, lifting the endometrial mucosa, sometimes causing atrophy and necrosis. They can be sessile, if they have a large implant base, or pedunculated if they are joined to the uterine wall by a peduncle; intramural (70%) develop in the thickness of the myometrium; subserosal (20%), when they develop just below the peritoneal serosa; also these myomas can be sessile or pedunculated.
Symptoms generally depend on the size, location and number of fibroids. About 20% of these lesions remain silent and are diagnosed during a visit or a follow-up pelvic ultrasound. The location of uterine myomas appears to be one of the main factors responsible for the frequency and severity of symptoms reported by patients. In fact, submucosal myomas are more frequently symptomatic, mainly causing menorrhagia, dysmenorrhea and infertility.
The most characteristic symptom of submucosal myomas is menorrhagia (30–40%). The abundant blood loss frequently leads to severe and disabling iron deficiency anemia, greatly influencing patient's lifestyle and often requiring medical therapy.
Although epidemiological data indicate that most women with myomas are fertile, numerous evidence suggests that myomas may interfere with fertility; especially the submucosal ones seem to exert most of the negative effects on reproductive outcome.
Myomas have also been associated with implant failure or premature termination of pregnancy due to focal vascular disorders of the endometrium, endometrial inflammation, secretion of vasoactive substances or local increase in androgens.
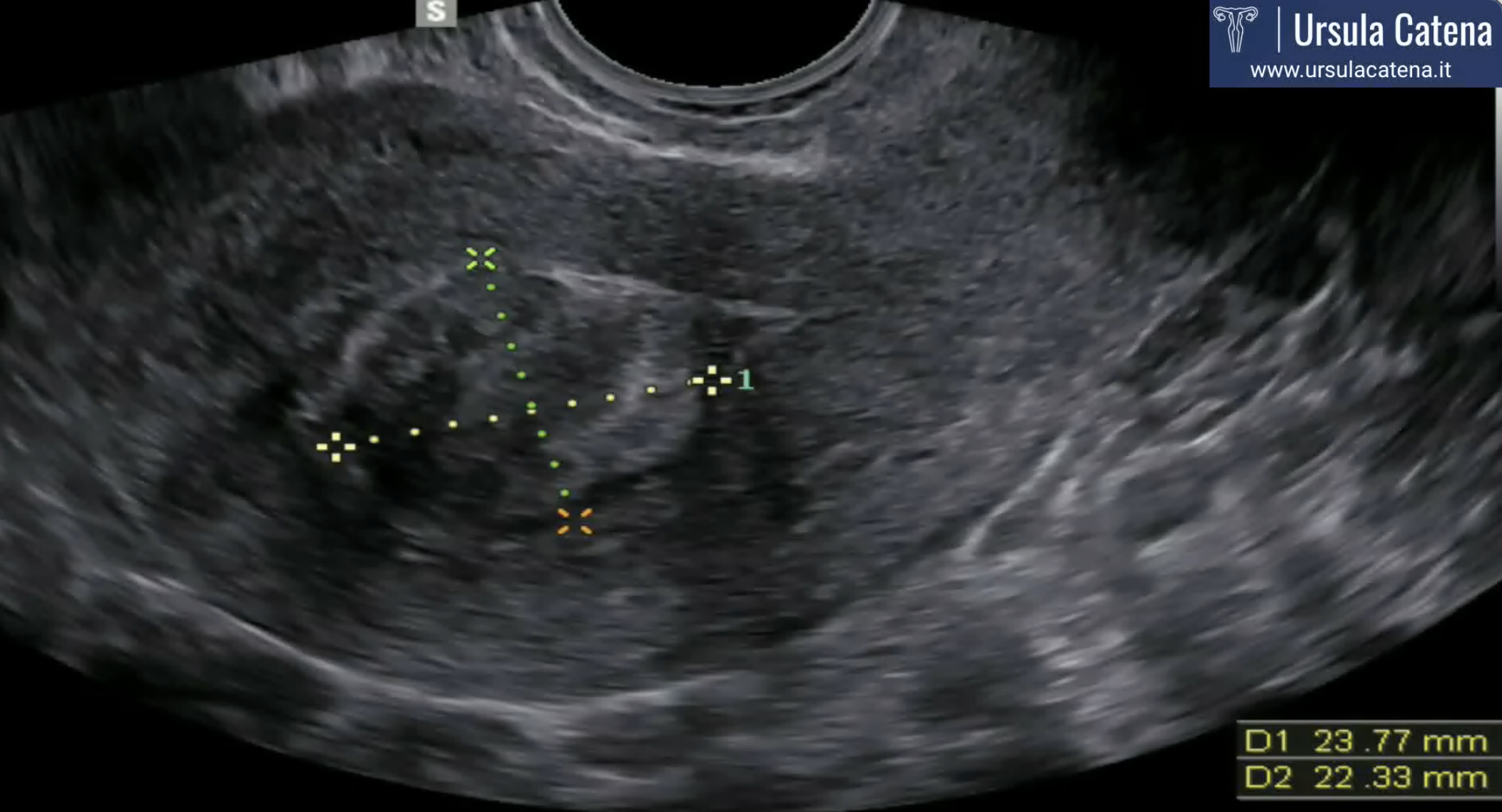
The diagnosis of uterine fibroids usually takes place through transvaginal pelvic ultrasound which shows a rounded neoformation with well-defined margins with an echogenicity that can widely vary in relation to the amount of muscle and connective tissue cells that make it up. Two-dimensional ultrasound and the most recent three-dimensional ultrasound techniques allow the study of the relationship between the myoma and the surrounding uterine cavity in such a way as to be able to correctly evaluate the subsequent surgical approach to be used (hysteroscopic, laparoscopic, laparotomy).
In case of significantly enlarged uterus, with multiple myomas, or in all cases where transvaginal pelvic ultrasound is technically difficult (eg obese patients), MRI can provide valuable informations.
Outpatient hysteroscopy is a fundamental step in the preoperative evaluation of uterine fibroids: in fact, in addition to ascertaining the presence of a possible submucosal myoma, it allows to study the uterine cavity and its relationship with the leiomyoma, in order to personalize the treatment. The parameters to be evaluated are: location, size, consistency, depth of myometrial invasion, vascularization, characteristics of the surrounding endometrium, presence of any associated pathologies.
In 2011 Munro et al. proposed the FIGO classification system (PALM_COEIN) for the causes of abnormal uterine bleeding in non-pregnant women of reproductive age. In this classification system a subclassification system of myomas was proposed based on the classification of submucosal myomas according to Wamsteker et al. (proposed in 1993) to which is added a characterization for intramural and subserosal myomas. According to this new classification, completely intracavitary lesions are defined as G0; G1 lesions have a component that is more than 50% intracavitary and an intramural component <50%; G2 lesions have an intramural component greater than 50%. G3 lesions are totally extracavitary but in contact with the endometrium. G4 lesions are intramural leiomyomas entirely within the myometrium, with no extension to the endometrial surface or serosa. Subserous leiomyomas (G5-7) represent the mirror image of submucosal leiomyomas: type 5 is at least 50% intramural, type 6 is less than 50% intramural, and type 7 is attached to the serosa by a pedicle (pedunculated myoma). A further category (G8) is reserved for leiomyomas that do not affect the myometrium at all and include cervical lesions, those that develop in round or broad ligaments and other so-called "parasites".
Hysteroscopic myomectomy is the best therapeutic option for the treatment of submucosal myomas (G0, G1, G2 and sometimes G3). It is often necessary that patient undergo medical preparation therapy before surgery which consists in the use of progesterone or GnrH analogues depending on the size of the pathology to be treated. Preoperative medical treatment is important because it makes the endometrium thin making the hysteroscopic removal of the myoma easier; it also reduces uterine bleeding allowing the correction of the anemia from which patient is usually affected and to face the surgery with higher hemoglobin levels; it can also help to reduce intraoperative bleeding and sometimes allows the fibroid diameter reduction prior to the surgery.
Hysteroscopic myomectomy is usually performed using the slicing technique, which consists of repeated and progressive passages of the cutting loop, according to the following modality: the cutting loop is brought behind the neoformation, the cutting current is then activated during the loop return movement to the rest position, in such a way as to obtain "slices" of tissue. The resection usually begins at the free margin of the myoma, then proceeding uniformly towards its implant base. During the leiomyoma resection, fragments gradually dissected and accumulated in the cavity will tend to interfere with the correct vision. It will therefore be necessary to remove them, which will be repeated several times during the intervention. This technique can be performed with a 26 Fr resectoscope (about 1 cm) after dilating the cervical canal, or with a 15 Fr miniresectoscope (about 5 mm) in diameter if the fibroid does not exceed 2-2.5 cm in diameter; in this case the mechanical dilation of the cervical canal can be avoided. Both of these instruments are based on the use of bipolar energy, an advanced electrosurgery system that allows the use of physiological solution as a means of uterine distension and which avoids the passage of current through the patient's body. In fact, the electric circuit, in case of bipolar energy use, closes on the loop of the instrument itself, without the need for the application of a discharge plate on the patient's body. For both instruments, we also have cold loops, more robust loops that allow mobilization and easier removal of the intramural portion of the myoma.
Another innovative technique for the removal of uterine myomas is intrauterine morcellation which involves the use of a mechanical tool, without energy, which "chops and sucks" the myoma.
In case of small myomas, myomectomy can also be performed in an outpatient setting with the help of 5 Fr bipolar electrodes.
Combined techniques are also possible to improve surgical outcomes and to personalize the intervention on each patient.
Depending on the number, size and depth of myometrial invasion, patient should always be informed of the possibility that the fibroid is not removed in a single surgical step, but that a second surgical time may be required. This step can sometimes coincide with the outpatient hysteroscopic control which is carried out after about 30-40 days from the primary procedure, during which small residues of pathology can be removed. In about 20% of cases a second time surgery under sedation have to be scheduled.
The surgeon's skill and experience, as well as the techniques used, certainly play an important role in the success of the procedure itself.
Look our photos